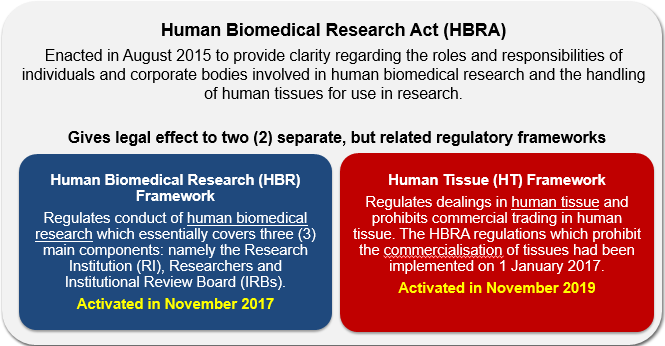
Human Biomedical Research Act (HBRA)
Information Pack for Researchers on the HBR Framework
The following materials provide researchers involved in the conduct of HBR activities a quick overview of the HBRA and its subsidiary legislations.
Section I: HBR Background & Framework 
Section II: Studies excluded from HBRA 
Section III: Restricted & Prohibited Research 
Section IV: HBRA and its implications on studies 
Resources
i) Human Biomedical Research Act 2015
ii) Human Biomedical Research Regulations 2017
iii) Human Biomedical Research (Restricted Research) Regulations
iv) Human Biomedical Research (Requirements for Appropriate Consent – Exemption) Regulations 2019
v) Human Biomedical Research (Tissue Banking) Regulations 2019
vi) Human Biomedical Research (Tissue Banking – Exemption) Regulations 2019
vii) Guidance on Consent Requirements
For more information, please refer to the MOH website.
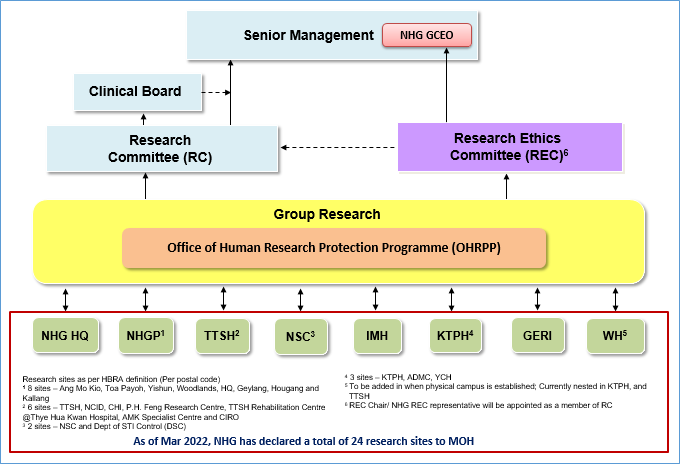
NHG Research Governance Framework
NHG Research Compliance Unit (RCU)
RCU ensures NHG’s compliance with the Human Biomedical Research Act (HBRA).
RCU oversees 5 key portfolios:
a. Secretariat for Research Institution (RI)
b. Secretariat for Research Data Oversight Committee (RDOC)
c. Secretariat for Tissue Compliance Committee (TCC)
d. Execution of Proactive Monitoring Framework
e. Secretariat for Responsible Conduct of Research (RCR)
NHG Research Institution (RI)
The NHG RI is responsible for ensuring that all human biomedical research conducted under its supervision and control is in compliance with the HBRA.
For the purpose of coordinating research across collaborative studies, the RI of the submitting/lead PI will be assumed as the lead RI.
About the RI Policy
The RI Policy outlines the functions and duties of NHG RI and duties of the NHG Principal Person In Charge (PIC) to fulfill the requirements of the HBRA, and describes the NHG research governance framework that enables the RI to meet the requirements of the HBRA and other relevant Acts and regulations applicable to research. This also outlines the roles and responsibilities of the following committees that provide oversight of human biomedical research (HBR) activities in NHG:
i) Research Committee (RC)
ii) Research Ethics Committee (REC)
iii) Tissue Compliance Committee (TCC)
iv) Research Data Oversight Committee (RDOC)
Get a copy of the NHG RI Policy here (NHG Intranet access required).
HBR Case Studies
The NHG RI shares good practices and negative examples using short case studies on a regular basis to help researchers understand the what-to’s and how-to’s of HBRA.
Click here to view/download the Case Studies. (NHG Intranet access required)
Restricted Human Biomedical Research (rHBR)
HBR involving human eggs/embryos or human-animal combination are restricted, and subject to tighter controls.
Restricted HBR requires obtaining approval from Institutional Review Board (IRB), Institutional Animal Care and Use Committee (IACUC) where applicable, and the Director of Medical Services, amongst others.
To find out more about rHBR application, click here.
Who should know?
NHG staff who wants to conduct Restricted Human Biomedical Research.
vi) Human Biomedical Research (Tissue Banking – Exemption) Regulations 2019



