Determine if Ethics Review is required & Meeting Dates
Research is defined as a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge. All research involving patients, staff, premises, or facilities of institutions under the oversight of NHG DSRB must be reviewed and approved by NHG DSRB prior to initiation.
This includes, but not limited to, clinical trials, epidemiology research, retrospective medical records review research, and genetic research.
Examples of Research Activities that Require DSRB Approval:
a. Case Series
b. Database Studies
c. Tissue Repositories
Examples of Research-Like Activities that May Not Require DSRB Approval:
a. Case Reports
b. Outbreak Investigations
c. Disease Management
d. Infection Control
For more information, please click HERE (NHG Investigators' Manual, Page 13)
DSRB Full Board Meeting Dates
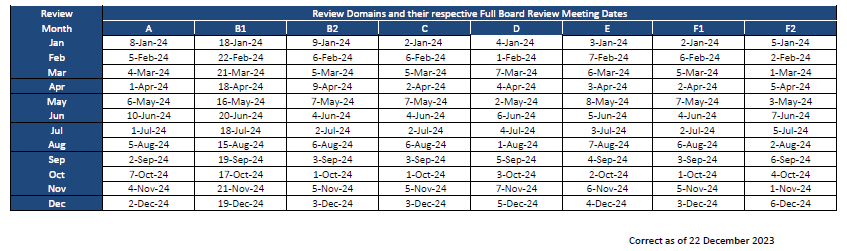
Please note that Full Board meeting dates may change without prior notice.
Kindly note that the DSRB submission deadline for Full Board studies is on the 15th day of the month or the next earliest working day if that day falls on a weekend.
This is with the exception of Domain B1 whereby the submission deadline for Full Board studies would be on the 1st working day of the month or the next earliest working day if it falls on a weekend.
Do note that applications should be submitted with sufficient lead time for the Department Representative and Institutional Representative to endorse prior to the submission deadline for the month.
Studies that fall into the Exempt and Expedited review categories will be reviewed by the Domain Chairperson at the weekly chair meeting.
