Who can be PI
Introduction to being a Principal Investigator
The Principal Investigator (PI) is an individual who assumes the authority and responsibility for the conduct of a research study. The PI has the authority to delegate responsibility to individual members of the research team; however, the PI is ultimately responsible for the overall conduct of the research study.
The PI(s) should have minimum ethical training to help them appreciate and apply the ethical principles underlying research to the day to day practice of research. Click here to find out more about the minimum ethical training requirements to be a PI.
This requirement is not solely for the purpose of the application to DSRB, as the PI has the responsibility for ensuring that the conduct of the research within NHG or its partner institutions is in compliance with Good Clinical Practice (GCP) and all other applicable guidelines and regulations.
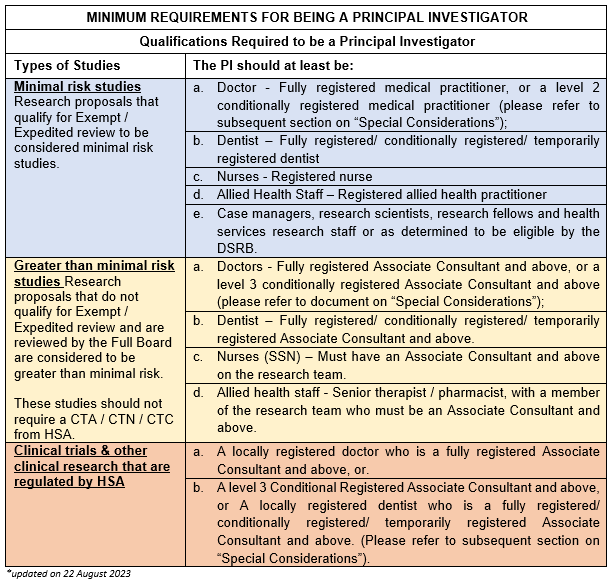
1. Who Can Be A Principal Investigator?
The PI should be a staff of NHG or partner institution for research conducted in NHG or partner institutions.

For more details on the minimum training requirements for being a PI of a research, please refer to the NHG Investigator’s Manual which is available here.
Special Considerations (see doc “Special Considerations”)
i. Visiting Consultants
ii. Conditionally Registered Medical Practitioners
iii. Conditionally / Temporarily Registered Dentist
Click here to download "Special Considerations" 
2. What other qualifications must the PI have?
The PI must be:
• Qualified by education,
• Training and experience to assume responsibility for the proper conduct of a research study and,
• Should meet all qualifications specified by the applicable regulatory requirements.
3. What are the qualifications for being a PI for a Clinical Trial?
Refer to the above table.
For more information regarding HSA requirements on who can be a PI, click here OR visit
https://www.hsa.gov.sg/clinical-trials/conducting
For more information regarding on Pharmacist being a PI -
1. Download a copy of the Regulations from https://sso.agc.gov.sg/SL/HPA2007-S332-2016
2. Download a copy of the guidance from https://www.moh.gov.sg/hpp/pharmacists/guidelines
4. What are the responsibilities of the PI?
The PI is responsible for promoting proper conduct of research by assuring adherence to protocol requirements, ensuring adequate resources to conduct the study, protecting the rights and welfare of participants, assuring the integrity of data generated at the site and directing the conduct of the research according to applicable regulations and guidance.
For more details on the responsibilities of the PI, please click here for the NHG PCR SOP 501-A02: Responsibilities of the Research Team
5. Is the PI able to apply for research grant?
The NHG Group Research promotes research in NHG through the administration of competitive grant calls via the bottom-up approach and the thematic.
Researchers who are interested to apply for research grants may click here.
Researchers are encouraged to refer to their Clinical Research Units/ Offices of their institutions for their own grants available.



