Human Tissue Management
The Human Biomedical Research Act (HBRA) came into effect on 01 November 2017. The Human Tissue Framework (HTF) was activated on 01 November 2019. Under this Framework, tissue banks must ensure that all tissue banking activities are in compliance with the HBRA HTF and its subsidiary legislations and conducted in accordance with the NHG Tissue Bank Policy, Proper Conduct of Tissue Bank (PCT) SOPs and institutional standards, and procedures.
Who will be impacted?
If you are involved in the collection, storage, procurement, importation, supply, provision or exportation of human tissue to facilitate future research, you may be subjected to the requirements of the HTF.
These may include, but are not limited to:
1) Clinical/ diagnostic laboratories that supply left-over/ waste tissue to researchers;
2) Researchers who collect tissue in excess for the sole purpose of storage for future research;
3) Persons in possession of tissue, who are not involved in research, but supply the tissue to other researchers.
Updates to the Governance of Leftover Tissue for Future Research
MOH has affirmed that upon study completion, any leftover human tissue to be stored for future research (research that has yet to obtain IRB approval) will need to come under the governance of the HTF.
If you are storing leftover tissue for future research, please refer to the table below for the required follow-up actions.
| Scenarios |
ICF Requirements for leftover tissue to be used in future research |
Is registration as a tissue bank with the Tissue Compliance Committee (TCC) Required? |
| You have completed your studies, and have some leftover human tissue. These tissue were collected before 1 Nov 2019. |
The ICF used to consent the donors of such tissue must at least have HBRA 12(2)(a), 12(2)(f) and 12(2)(i) elements. |
Yes. Your tissue must be registered with TCC.
Please submit the TCC tissue bank application form.
Otherwise, if the tissue had been made non-identifiable prior to 1 Nov 2019, you can submit using the Legacy Human Biological Materials Declaration form instead. |
| You have completed your studies, and have some leftover human tissue.These tissue were collected from or after 1 Nov 2019. |
The ICF used to consent the donors of such tissue must have all HBRA 12(2) elements. |
Yes. Your tissue must be registered with TCC.
Please submit the TCC tissue bank application form. |
| You have an ongoing study, and intend to store leftover tissue for future unspecified research. |
The ICF that you use to consent current subjects must include all HBRA 12(2) elements |
If researchers still require the tissues for analysis, even after IRB’s acknowledgment of study closure, such tissues could be retained and used within 12 months of IRB acknowledgment for study closure. After which, the tissues should be discarded, transferred to a tissue bank or an IRB approved study or registered with the NHG TCC. |
The above requirements will not apply to leftover tissue stored for future research that has received IRB approval. This is because such future research demonstrates clear research intent that can be governed under the Human Biomedical Research (HBR) Framework. Hence, HTF requirements need not apply.
You may refer to the following resources for more details.
1. March Issue 01-2021 (MOH Update on the Governance of Leftover Tissue)
2. June Issue 02-2021 (MOH Update on the Governance of Leftover Tissue (Part 2))
3. NHG Guidance Document to Store and Use Leftover Human Tissue for Future Research
NHG Governance Structure for Human Tissues
NHG is registered under MOH as a single "Mothership" Tissue Bank. Under this governance structure, each NHG institution has established an Institutional Tissue Bank Committee (ITBC) to oversee institutional-level tissue banking activities. For more information on the framework, click here *.
About NHG Tissue Compliance Committee (TCC)
The NHG TCC was formed to advise on the set up, conduct and monitoring of institutional human tissue banks. The TCC is chaired by A/Prof Leong Khai Pang (Senior Consultant, TTSH) and comprises representatives from each NHG institution.
For the list of TCC members, please click here *.
Tissue Bank Registration
1) Download and complete the "Tissue Bank Application Form*"
2) Seek endorsement from your Head of Department (HOD) or equivalent
3) Seek endorsement from your Institutional Tissue Bank Committee (ITBC) or equivalent
4) Submit the completed form to the NHG TCC Secretariat (NHGTCCSecretariat@nhg.com.sg)
Fig. 1 – Tissue Bank Application Process in NHG
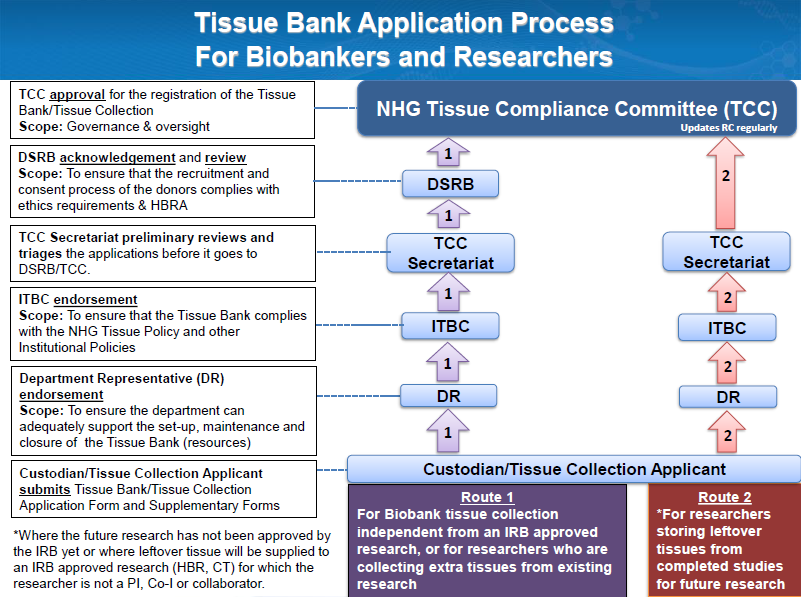
For collection of human tissue that is for the sole purpose of bio-banking and is not linked to any IRB-approved research, custodians must also complete the "NHG Tissue Collection Form".
Minimum Training for Tissue Bank Custodians/Tissue Collection applicants and team members
The TCC has compiled a summary of the legal and institutional requirements into a short online course titled “Human Tissue Framework Course” to help NHG Tissue Bank Custodians/Tissue Collection Applicants and team members familiarize with the HBRA HTF. The completion of this course will be mandatory for all NHG Tissue Bank Custodians/ Tissue Collection Applicants and team members.
The training certificate of the Custodian/Tissue Collection Applicants must be submitted to the TCC during Tissue Bank and or Tissue Collection registration. A copy of the training certificate should also be retained by the Tissue Bank/Tissue Collection team as proof of understanding the HBRA HTF requirements and NHG Tissue Bank policies.
From 25 Mar 2022, the HTF Minimum Training requirements (HTF Online Course) will be extended to SAF staff/doctors appointed in NHG and involved in Tissue Banking Activities.
There is no expiry to the Human Tissue Minimum Training Certificate.
Click here* for details of the NHG HTF Course. For more information, please click here.
Tissue Review Forms and Templates
i) Tissue Bank Application Form* – This form is submitted to the TCC to register a Tissue bank
ii) Tissue Collection Application Form* – This form must be submitted to the TCC if a NHG staff intends to actively recruit donors independent of an IRB approved research for the sole purpose of collecting tissue to facilitate future research
iii) Declaration of Compliance to HBRA on Handling of Legacy Human Biological Material (HBM) – This form is required for NHG staff who are in possession of Legacy HBM and intends to store or use them for future research from 01 November 2019
iv) Other Supplementary Forms* – For reporting of Non-compliances, Amendments, Continuing Reviews, Serious Adverse Events (SAEs), & Untoward Occurrences
NHG Tissue Bank Policy, PCT SOPs and Templates
There are a set of policy, guidelines and templates developed by NHG Tissue Compliance Committee to provide detailed procedures on conducting tissue banking activities in accordance with applicable guidelines and regulations (e.g. HBRA). You may adapt and modify these guidelines and templates to suit your individual tissue banking activities.
A list of PCT SOPs and Templates can be downloaded on this page.
Other Resources
i) Tissue Banking Regulations 2019
ii) Tissue Banking - Exemption Regulations
iii) Requirements for Appropriate Consent - Exemption Regulations
iv) Quick Guide on Setting Up and Managing a Tissue Bank
v) Guidance on Prohibition Against Commercial Trading of Human Tissue under HBRA - February 2017
vi) Human Tissue Framework Course
vii) Human Tissue Framework FAQ
viii) Communication Session on the Human Tissue Framework and Updates to HBR Regulations (02 Dec 2019)*
Tissue Compliance Circular
In an effort to educate the NHG research community on the HTF, the Tissue Secretariat have initiated a publication series called “Tissue Compliance Circular” for tissue bank custodians/TCAs and individuals involved in tissue banking activities.
Click here to view/download the Circulars. (NHG Intranet access required)
Contact
NHG TCC Secretariat – NHGTCCSecretariat@nhg.com.sg
* NOTE: These document downloads can only be accessed when you have direct access to the NHG Intranet at the time of downloading. You may also be required to login with your NHG ADID.
These documents are strictly for internal circulation among NHG Staff members and Authorized personnel only.

