Quality Improvement Initiatives
In 2014, the NHG Office of Human Research Protection Programme (OHRPP) partnered the TTSH Kaizen Office in a Quality Improvement (QI) project to look into improving the turnaround times for New DSRB Applications that are reviewed via the Full Board review route.
Different stakeholders (DSRB administrators, Principal Investigators, study administrators) participated in the QI project to give their inputs to help the team identify possible root causes of delays in the DSRB review process. The team also studied references from other local and international IRBs. After several brainstorming and consultation sessions with the Kaizen Office Facilitators and the Process Owners, the team agreed on several action plans that have been implemented in phases since 2015.
(A) Enhancements to the DSRB Application Form
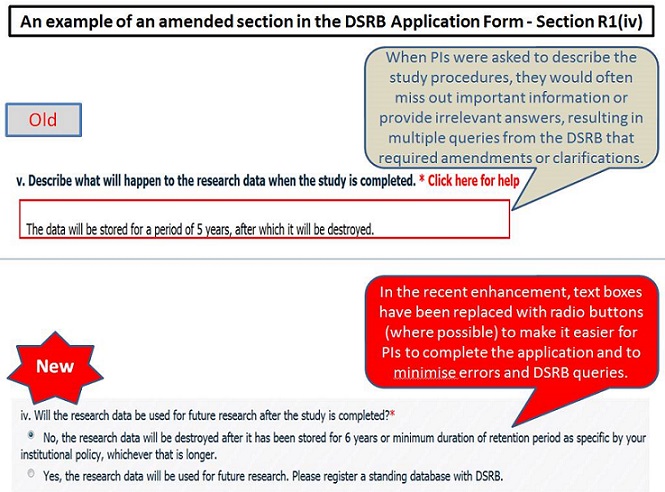
The DSRB Application Form has been enhanced, primarily to:
(i) remove repeated questions,
(ii) provide greater clarity on the intention of the questions, and
(iii) improve the ease of selecting the appropriate answer (e.g. using radio buttons or checkboxes instead of free-text textboxes).
Click here to learn more about the key enhancements to the DSRB Application Form.
(B) Auto-Reminders to PIs & Study Team Members to Respond to DSRB Queries
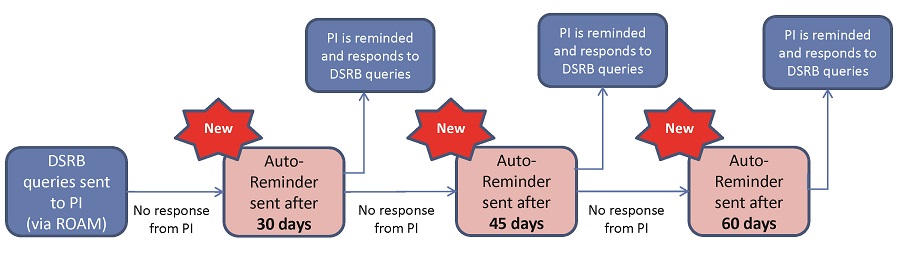
PIs & Study Team Members would receive reminders to respond to outstanding DSRB queries for their new studies.
(C) Improvements to the NHG Research Website
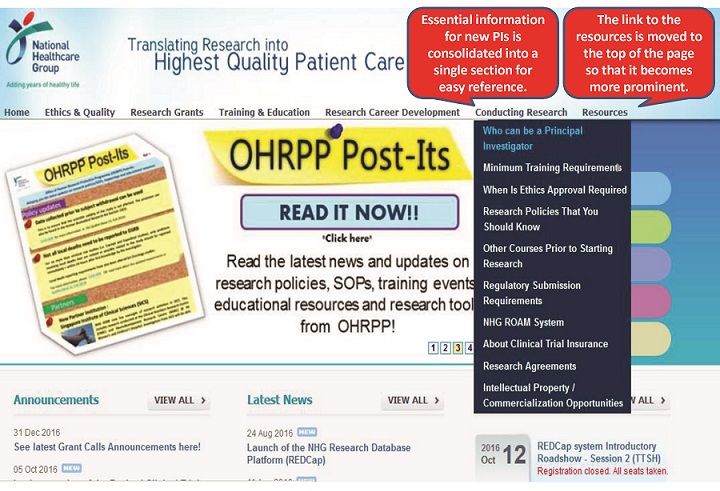
It is now easier for PIs & Study Team Members to navigate and locate the resources to assist them with preparing their DSRB applications.
(D) Survey on DSRB Query & Review Process
Selected PIs & Study Team Members were invited to participate in an online survey to rate their experience with the DSRB review and query process. They were also asked to provide examples of areas that were perceived as barriers or queries that were unclear. Their responses were used by the DSRB administrators to refine the way that queries are being drafted.
(E) Training of Institution-Based Resource Persons (IRPs)
Staff across various departments in TTSH, NHG Polyclinics and KTPH were identified and trained as part of a pilot training programme to be well-versed in the ethics submission requirements, so that investigators and administrators can have access to local and more effective support for the submission of good quality DSRB applications. Currently, 29 staff have successfully completed the training programme. Investigators are strongly encouraged to contact their respective IRPs for guidance in their ethics submissions or responses to DSRB queries.
Click here to download the list of trained IRPs.
Feedback or Comments
We would like to thank all our stakeholders who have provided valuable feedback to us. As we continue to roll out the action plans and monitor those which have been initiated, we seek your patience, support and continuous engagement to make our QI initiatives a success together.
If you have any feedback or comments, please write to:
Ms Ng Hwee Hian
Deputy Director
NHG Office of Human Research Protection Programme
Email Address: hwee_hian_ng@nhg.com.sg



